How Does Fluxguard Help Maintain Pharmaceutical Compliance and Drug Safety?
Risk & compliance, legal, and labeling teams operating in the healthcare and pharmaceutical space rely on Fluxguard’s website change detection technology to perform continuous surveillance of the regulatory landscape.
Reduce risk related to missing pharmaceutical regulatory changes and innovator labeling changes.
Comprehensively collect pharmaceutical regulatory intel efficiently and cost-effectively.
Seamlessly turn regulatory insights into actions that decrease response time and increase overall revenue.
Stay abreast of changes from the US Food and Drug Administration, the European Medicines Agency, and any regulatory body that impacts your work.
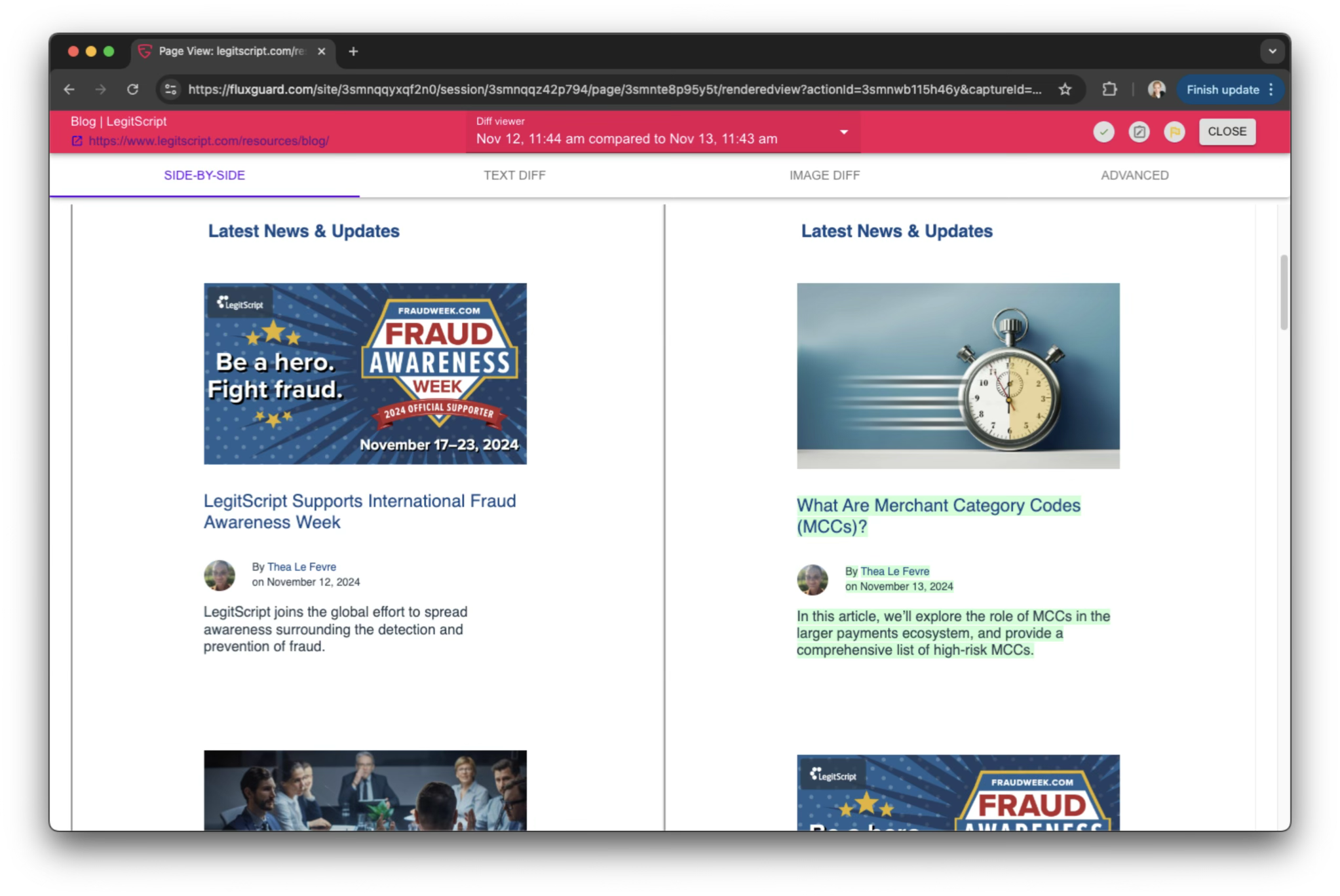
Track Regulatory Changes as They Happen
Stay abreast of changes to pharmaceutical labels and other requirements with accurate, timely, and comprehensive change detection and AI-powered alerts.
Why Use Fluxguard?
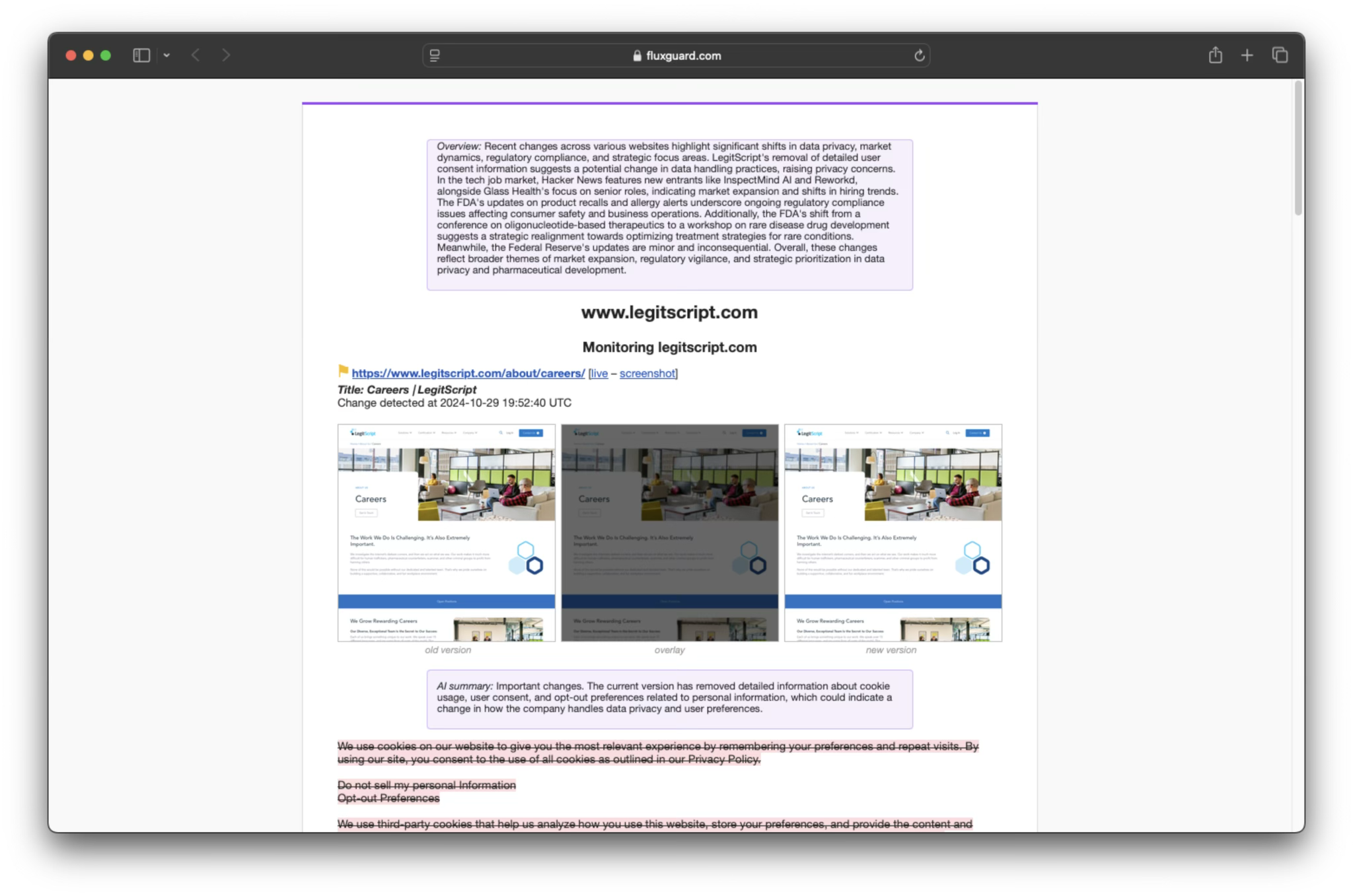
The rules and regulations that govern the pharmaceutical space are often buried within websites that require many clicks to navigate, and once located can be complex to understand. With Fluxguard you can:

Pharmaceutical Compliance Software Powered by AI Technology
Engineered in partnership with the US Air Force, Fluxguard automates risk detection from subtle digital clues and resistant data.
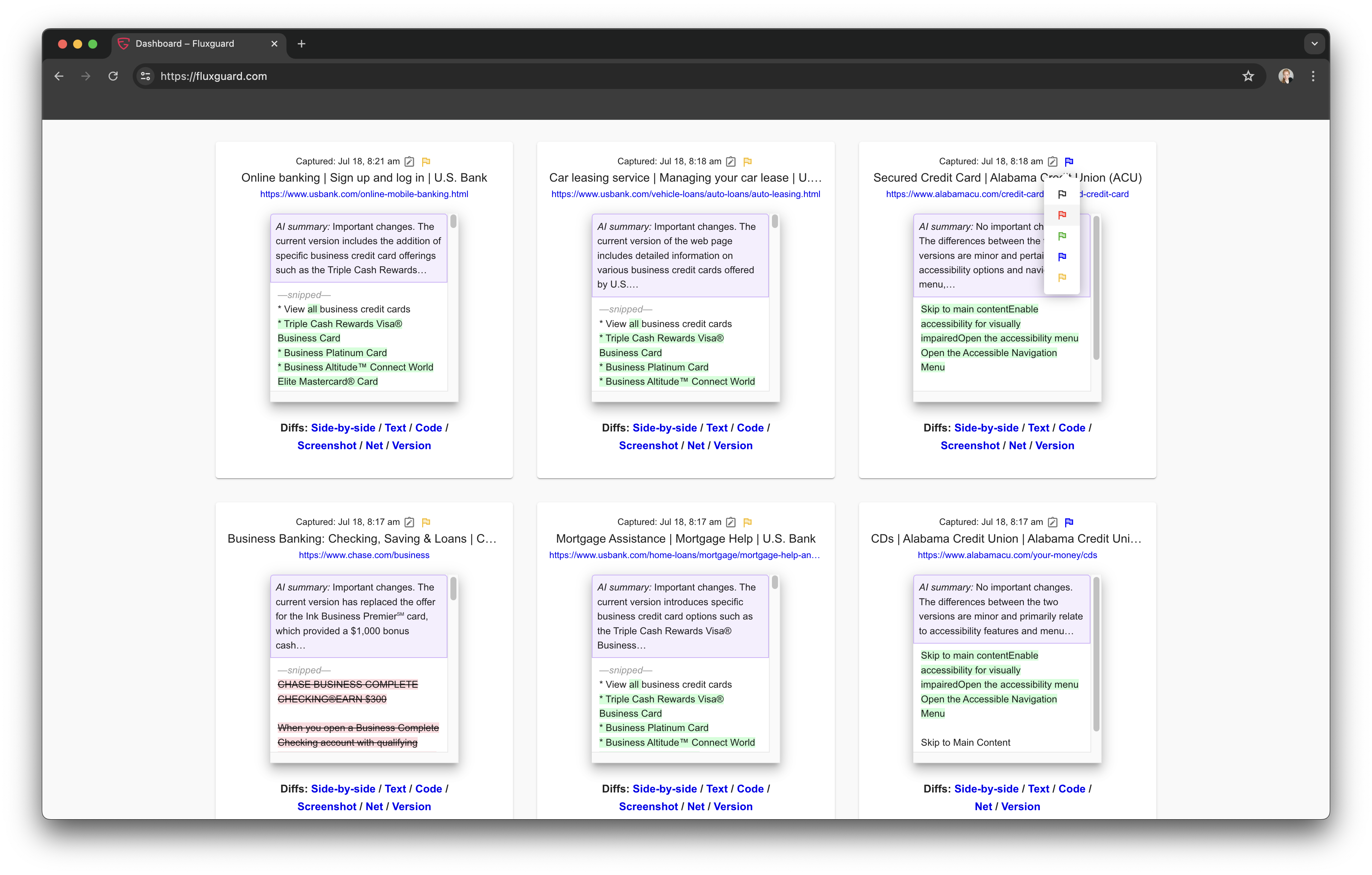
Here’s How Fluxguard Works
Crawls and Collects
Fluxguard first obtains data from often-complex web sources.
Evaluates and Prioritizes
Fluxguard then determines relevance to the customer’s rules and needs.
Transforms and Enriches
Next, Fluxguard uses change monitoring, ad hoc rules, and AI to tailor insights to each customer.
Integrates and Acts
Finally, Fluxguard dovetails results into your ecosystem when and where you need it.